Introduction to the Liquid Biopsy Market
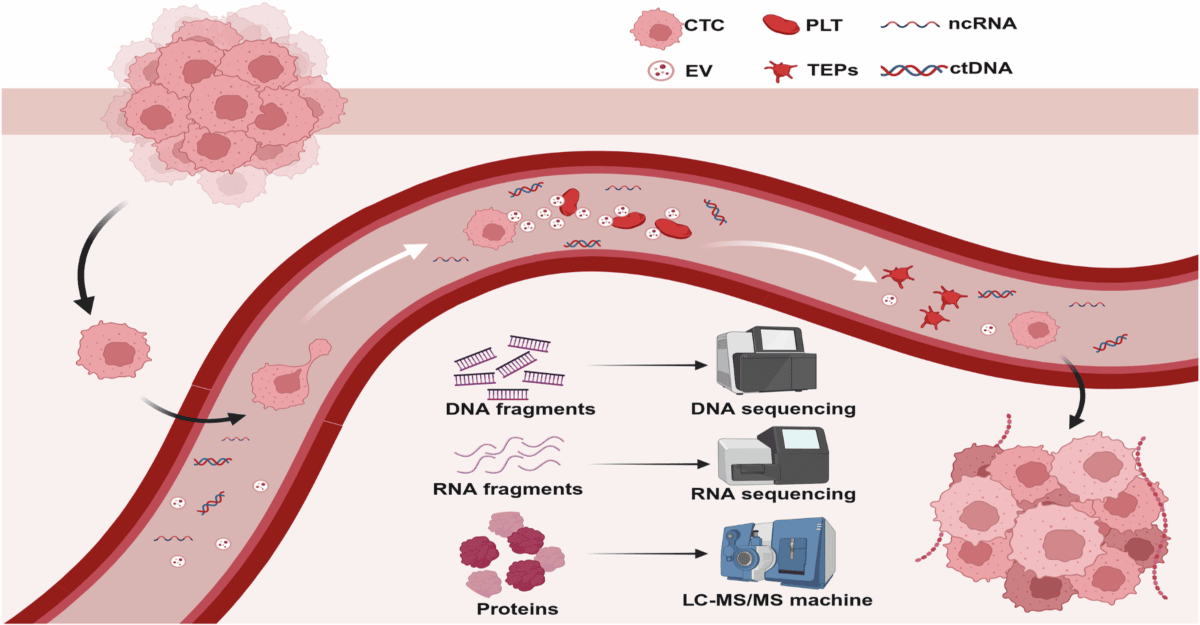
The liquid biopsy market represents a revolutionary shift in cancer diagnostics and treatment monitoring, capitalizing on the rapid advancements in molecular biology and genomic medicine. A liquid biopsy is a minimally invasive test that analyzes circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), or other tumor-related components found in bodily fluids such as blood, urine, or saliva. Unlike conventional tissue biopsies, which require surgical procedures to acquire samples, liquid biopsies offer a more accessible and safer method for obtaining vital information about the tumor’s genetic makeup.
The significance of liquid biopsies in the context of cancer diagnosis cannot be overstated. They enable early detection of malignancies, facilitate real-time monitoring of treatment efficacy, and provide insights into the tumor’s evolving behavior without the need for repeated invasive procedures. This non-invasive approach is particularly beneficial for patients who may be frail or have advanced disease, thus enhancing their quality of life and treatment experiences.
As the liquid biopsy market continues to evolve, its growth can be attributed to increasing demand for personalized medicine and targeted therapeutics. Regulatory bodies are recognizing the importance of these techniques, paving the way for more robust clinical validations and commercial applications. Additionally, the development of advanced technologies, such as next-generation sequencing (NGS) and digital PCR, bolsters the capabilities of liquid biopsy assays to detect and quantify minimal residual disease.
The potential benefits of liquid biopsies over traditional tissue biopsies are increasingly evident. Not only do they minimize patient discomfort and risk, but they also allow for comprehensive biomarker analysis that can enhance decision-making in treatment strategies. Overall, the growing acceptance and implementation of liquid biopsies mark a pivotal movement toward more effective and patient-centered cancer care.
Market Segmentation: Products, Applications, and Cancer Types
The global liquid biopsy market is segmented into three major categories: products, applications, and cancer types, each of which plays a pivotal role in the overall growth and advancement of this innovative diagnostic approach. In terms of products, the primary offerings include liquid biopsy assays and kits which facilitate the isolation and analysis of circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes. These products are essential tools enabling healthcare professionals to obtain vital information regarding a patient’s cancer status without the need for invasive tissue biopsies.
Applications of liquid biopsy are diverse and impactful, prominently featuring early detection, treatment monitoring, and disease recurrence assessment. Early detection remains a key driver as it allows for timely intervention, significantly improving patient prognosis. Furthermore, liquid biopsies provide real-time insights into treatment response, guiding oncologists in personalized medicine decisions, ultimately supporting the optimization of therapeutic strategies. These advances are crucial in managing chronic cancer conditions and reducing patient morbidity.
In addition to products and applications, various cancer types represent a significant aspect of the market segmentation. The liquid biopsy market has targeted numerous malignancies, including but not limited to breast, lung, and colorectal cancers. These prevalent cancers exhibit a substantial and growing need for effective screening and monitoring solutions. Notably, the market for lung cancer liquid biopsies has experienced remarkable growth due to increasing incidences and a rising emphasis on early detection strategies. Similarly, in the context of breast cancer, personalized treatment approaches facilitated by liquid biopsies have gained traction, enhancing the therapeutic journey for patients.
The interconnection of these segments is driving rapid advancements in the liquid biopsy market, with increasing investment in research and development further propelling growth trends. As these innovations continue to unfold, the future landscape of cancer diagnosis and management appears promising, transforming patient care in profound ways.
Regional Analysis and End-User Insights
The global liquid biopsy market exhibits significant variations across its geographical landscape, with notable distinctions arising from regional dynamics and end-user demands. In North America, particularly the United States, the market is propelled by advanced healthcare infrastructure, increasing prevalence of cancer, and a robust focus on research and development. The presence of leading companies and biopharmaceutical firms further bolsters this market, facilitating the adoption of liquid biopsy technologies in hospitals and laboratories.
Europe, too, demonstrates a dynamic liquid biopsy market, spurred by rising government initiatives to enhance cancer diagnosis and treatment. Countries such as Germany, France, and the UK are at the forefront, with substantial investments being poured into research institutions dedicated to developing innovative liquid biopsy methods. Moreover, the emphasis on personalized medicine is significantly shaping the market landscape, enhancing the growth potential of liquid biopsy applications across various medical sectors.
The Asia-Pacific region showcases a different set of market dynamics. Rapid economic development and an increase in healthcare expenditure are leading to a growing demand for advanced diagnostic methods, including liquid biopsy. The region is anticipated to experience significant growth as countries like China and India increase research collaborations and strive to improve their healthcare systems. Hospitals and laboratories in these countries are gradually adopting liquid biopsy techniques to streamline cancer diagnostics.
Regions outside of North America, Europe, and Asia-Pacific, though smaller in market share, present emerging opportunities. The Middle East and Africa, with their diverse healthcare challenges and increasing investments in healthcare infrastructure, represent a budding market for liquid biopsy technologies. As awareness grows among end-users, including laboratories and research institutions, the potential for liquid biopsies in improving diagnostics and treatment choices becomes increasingly evident.
Competitive Landscape: Major Companies and Future Trends
The liquid biopsy market has experienced significant evolution over the past decade, largely driven by advancements in technology and a growing interest in non-invasive diagnostic methods. Key players in this competitive landscape include companies such as Guardant Health, Biocept, and Foundation Medicine, all of which have established strong brand identities through innovative product offerings and strategic partnerships. Guardant Health, for example, is renowned for its Guardant360 test, which allows comprehensive genomic profiling through blood samples. This innovation has positioned the company as a leader in precision oncology.
In addition to these specific products, companies are increasingly focusing on research collaborations and strategic alliances to expand their market reach and enhance their technological capabilities. Partnerships between academic institutions and biotech firms are becoming more commonplace, facilitating the acceleration of liquid biopsy technologies from laboratory research to clinical application. Such collaborations have been instrumental in harnessing data analytics and artificial intelligence, necessary for the implementation of precision medicine.
Looking to the future, several trends are poised to shape the liquid biopsy landscape between 2026 and 2034. For one, the integration of advanced molecular techniques such as next-generation sequencing (NGS) will likely enhance the accuracy and reliability of liquid biopsy tests. Furthermore, the rising prevalence of cancer and other chronic diseases necessitates the continuous evolution of diagnostic methods, propelling research into innovative solutions.
However, challenges remain, including regulatory hurdles and market entry barriers that may impede the growth of emerging players. Companies must navigate the complex landscape of clinical validations and reimbursement policies to ensure that innovative solutions are not only developed but also adopted into standard clinical practice. These dynamics indicate a robust and competitive future for the liquid biopsy market, with potential for growth contingent on strategic adaptability and technological advancements.